
Ultrasonic Energy Transfer for Non-Invasive Implant Charging
The development of reliable wireless charger systems represents a significant advancement in medical device charging technology, particularly for implantable devices requiring regular power replenishment. Traditional battery replacement surgeries for pacemakers and neural stimulators pose unnecessary risks and discomfort for patients. Emerging ultrasound-based solutions offer a promising alternative for transcutaneous power transfer, potentially transforming how we maintain critical health technologies without repeated surgical interventions. As a battery longevity researcher who has witnessed firsthand how temperature extremes degrade performance (like when my navigation app failed during a summer road trip due to overheating), I recognize that the same principles of thermal management apply equally to medical implants: a cool battery is a long-lived battery.
FAQ Deep Dive: Ultrasonic Energy Transfer for Medical Implants
What is ultrasonic energy transfer and how does it work for medical devices?
Ultrasound-based wireless power transfer (UWPT) utilizes high-frequency sound waves (typically 20 kHz to 10 MHz) to transmit energy through biological tissues to implanted devices. Unlike electromagnetic induction used in smartphone wireless charger systems, ultrasonic energy transfer converts electrical energy into acoustic waves via a piezoelectric transducer outside the body. These waves travel through tissue and are converted back to electrical energy by a receiver implanted with the medical device.
The technology operates on principles similar to medical ultrasound imaging but optimized for power delivery. Advanced implementations, such as the sandwich-structured piezoelectric energy harvester developed by DGIST researchers, capture residual ultrasound energy with multiple layers to boost efficiency by over 20% compared to conventional designs. This dual-layer approach allows the system to fully charge commercial batteries within two hours, even when implanted deep within the body.
Keep it under 40°C when possible, a principle that applies equally to your smartphone and life-sustaining medical implants.
How does ultrasound compare to RF and electromagnetic induction for implant charging?
Radio frequency (RF) and electromagnetic induction methods face significant limitations for deep-tissue applications:
- Tissue absorption: RF energy is substantially absorbed by biological tissues, limiting penetration depth and potentially causing localized heating.
- Safety thresholds: Electromagnetic fields must remain below specific absorption rate (SAR) limits to prevent tissue damage, constraining power delivery.
- Alignment sensitivity: Electromagnetic induction requires precise coil alignment, which becomes problematic for deep implants.
Ultrasound overcomes these limitations due to its physical properties. As noted in KIST research, ultrasound waves experience less absorption in biological tissues compared to RF, allowing for deeper penetration with lower thermal impact. Studies confirm ultrasound can deliver 7 mW of power at 3 cm depth from the skin (sufficient for many low-power medical devices) while maintaining tissue temperatures within safe limits. This makes ultrasonic energy transfer particularly valuable for deep-tissue applications where conventional methods fail. For a broader look at alignment-free methods outside the body, see our resonant wireless charging overview.
What safety considerations exist for ultrasound-based implant charging?
While ultrasound has been safely used in medical imaging for decades, power transfer applications require careful thermal management. The FDA limits tissue temperature increases to 2°C for prolonged exposure, requiring precise power regulation. Research from Penn State demonstrates that dual-energy harvesting systems can operate within established safety limits for human tissue while delivering significantly more power than single-source approaches.
Critical safety parameters include:
- Intensity limits: Medical ultrasound typically operates below 720 mW/cm² to prevent tissue damage
- Thermal thresholds: Continuous monitoring ensures tissue temperatures remain below 41°C
- Exposure duration: Duty cycling prevents sustained heating at any single location For medical-specific standards and risk controls, explore our medical wireless charging safety guide.
Practical implementation must also address coupling challenges (the air gap between skin and transducer significantly reduces efficiency). Modern approaches use flexible polymer interfaces to eliminate the need for ultrasound gel, making home use feasible without professional assistance.
How efficient is ultrasound-based charging compared to other methods?
Recent advancements have dramatically improved the efficiency of ultrasonic energy transfer systems. The KIST-developed biocompatible receiver achieves power conversion rates sufficient to continuously power low-power medical devices, delivering 20 mW of power at a 3 cm distance underwater. This represents a significant improvement over earlier generations.
Comparative efficiency metrics:
- Electromagnetic induction: 40-60% efficiency at shallow depths (≤1 cm), rapidly declining with depth
- RF transmission: 10-30% efficiency for deep implants due to tissue absorption
- Ultrasound transmission: 30-50% efficiency at 3-5 cm depths, with less depth-dependent degradation
The Penn State dual-energy harvester represents a breakthrough, generating 300% higher power than state-of-the-art single-source devices by simultaneously harvesting both magnetic field and ultrasound energy. This hybrid approach could enable millimeter-sized, battery-free implants that were previously impossible due to power constraints.
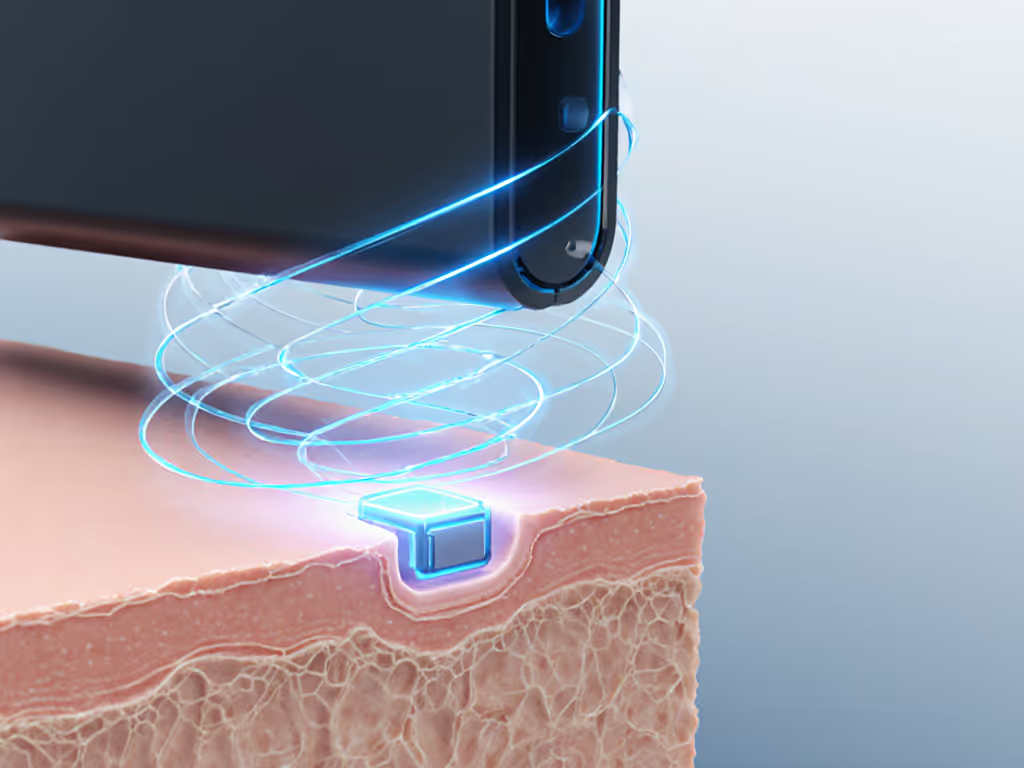
What are current limitations of ultrasound charging for implants?
Despite promising advances, several challenges remain before widespread clinical adoption:
- Alignment requirements: Precise targeting of ultrasound beams requires sophisticated beam-forming algorithms, though research from TTP indicates automated systems using advanced focusing techniques are nearing practical implementation
- Power density constraints: While sufficient for many applications, current systems cannot rapidly charge high-power devices without exceeding safety thresholds
- User interface challenges: Developing intuitive home-use systems that eliminate the need for clinical expertise in transducer placement
- Standardization gap: Unlike consumer Qi standards, medical ultrasound charging lacks universal protocols for interoperability
These limitations highlight why thermal management remains critical, pushing power delivery too aggressively risks tissue damage. My own experience with a bargain fast-charging mount that overheated my phone serves as a reminder that safeguards must precede speed, especially when human health is at stake.
What future developments are expected in this field?
Several promising research directions could transform non-invasive charging for medical implants:
- Miniaturized receivers: DGIST and KIST researchers are working on sub-millimeter harvesters that could power microscopic implants
- Smart beam forming: Advanced algorithms that automatically detect and focus energy on implanted devices without manual alignment
- Hybrid systems: Combining ultrasound with lower-frequency magnetic fields (as demonstrated by Penn State) to maximize power delivery within safety limits
- Closed-loop thermal management: Real-time temperature monitoring that dynamically adjusts power delivery to maintain safe operating conditions
The convergence of these technologies could eventually enable truly maintenance-free implants that operate for decades without battery replacement surgery. As research continues to optimize the balance between power delivery and thermal safety, these systems will increasingly incorporate the principle that protecting the energy storage system ensures long-term reliability.
Conclusion: A Path Toward Truly Maintenance-Free Medical Implants
Ultrasound-based transcutaneous power transfer represents a significant leap toward eliminating the need for battery replacement surgeries in implantable medical devices. While current implementations show impressive promise, ongoing research into efficiency improvements and thermal management will determine their clinical viability. The field would benefit from adopting the same rigorous safety standards that govern consumer wireless charging, after all, the fundamental truth that "protect the pack, and performance naturally lasts the distance" applies equally to medical implants as it does to our everyday devices.
For those interested in the technical evolution of this field, I recommend following publications from the Korea Institute of Science and Technology (KIST) and DGIST researchers who are publishing regular updates on efficiency improvements. The convergence of materials science, acoustic engineering, and battery management principles will ultimately determine which technologies transition from laboratory success to clinical standard of care.




